Ferriprox dosing optimization
Your doctor may adjust your dose to minimize GI upset when first starting therapy and over time to help you receive the full benefit of Ferriprox treatment.1
Iron level
Talk to your doctor about whether your iron level is controlled and if your Ferriprox dose should be increased from 75 mg/kg/day to 99 mg/kg/day to improve chelation or to help you reach iron level goals.



Increasing the dose of Ferriprox from 75 mg/kg/day up to 99 mg/kg/day may improve efficacy in iron chelation.1,2
Weight change
Ferriprox is dosed according to your weight. This means if you lose or gain weight your doctor may need to adjust your dose to ensure you receive optimal iron chelation.3
Talk to your doctor to see if you are on the optimal Ferriprox dosage.
Most common side effects
Be sure to tell your doctor about side effects that keep you from taking your medication as prescribed like:1
- gastrointestinal side effects (i.e., nausea and vomiting)
- joint pain
- bone pain
- stomach area (abdominal) pain
- pain in arms or legs
- back pain
- mouth and throat pain
- cough
- fever
- headache
Your doctor may need to adjust your dose to help lessen these or other side effects.
Choice of formulations
Ferriprox offers a choice of oral chelation formulations1†:
Insert text here...
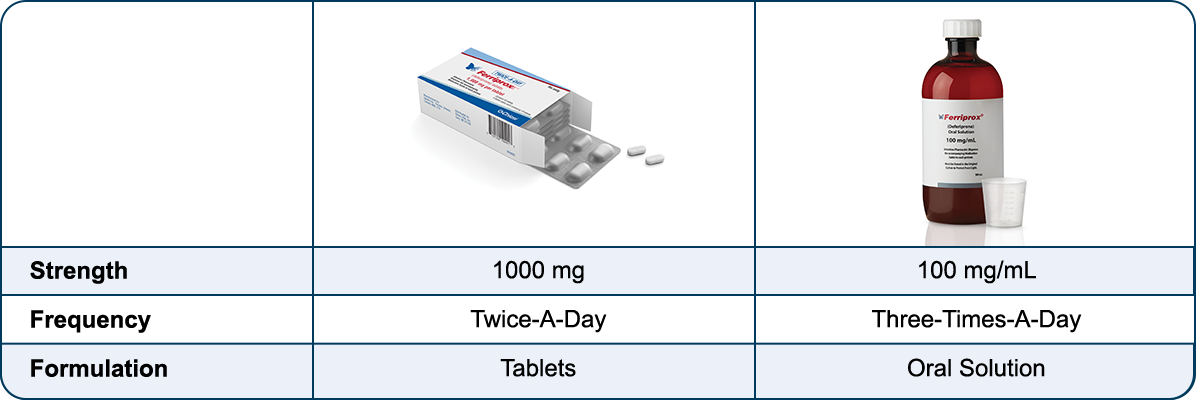


† 500 mg and 1000 mg Three-Times-A-Day tablets are still available. Talk to a Chiesi Total Care pharmacist for more information: 1-866-758-7071.
IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
References: 1. Ferriprox® (deferiprone) Prescribing Information. Chiesi, November 2021. 2. Binding A, et al. Deferiprone exerts a dose-dependent reduction of liver iron in adults with iron overload. Eur J Haematol 2019;103(2):80-87. 3. Coates TD, Wood JC. How we manage iron overload in sickle cell patients. Br. J Haematol 2017;177(5):703-16. 4. Evidence-based management of sickle cell disease: Expert panel report, 2014. National Heart Lung and Blood Institute. Accessed online January 13, 2021 at: https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease 5. Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol 2015;11(3):1171-71 6. Sundaram N, Bennett M, Wilhelm J et al. Biomarkers for early detection of sickle nephropathy. Am J Hematol 2011;86(7):559-66.
