Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Iron accumulation is toxic to the organs
Iron removal (chelation) is important for you if you receive blood transfusions for the treatment of sickle cell disease.
It is important to keep track of all of your blood transfusions, including the ones you might receive in the ER.
Each blood transfusion adds 200 to 250 mg of iron to the body.
One unit of blood can deliver >100X the normal daily iron absorbed
In sickle cell disease, expert guidelines say you should start iron chelation when you:



Ferriprox was studied in a 1-year, controlled non-inferiority clinical trial of 185 people with sickle cell disease. 122 patients received Ferriprox and 63 received deferoxamine over 1 year.
In the clinical trial, the mean decrease in liver iron concentration (LIC) from baseline was -4.13 mg/g dw ± 0.50 after 1 year of treatment with Ferriprox compared to 4.38 ± 0.59 mg/g dw for deferoxamine1
People treated with Ferriprox experienced an additional 30% reduction in liver iron level after 3 years of therapy. LIC dropped from 14.93 mg/g dw at baseline to 10.45 mg/g dw after 3 years of treatment.1
In a 2018 review of clinical trials, sickle cell patients with iron overload† had higher rates of:2
DEATH 64% compared to 5% for those without iron overload
ORGAN FAILURE 71% compared to 19% for those without iron overload
PAIN EPISODES 64% experienced ≥3 pain episodes per year compared to 38% for those without iron overload
Another clinical study in 199 transfused people with sickle cell disease compared to 64 non-transfused people with sickle cell disease also showed an increase in the number of:
HOSPITAL STAYS 4.1 hospitalizations per year compared to. 2.1 for non-transfused people (p<0.001)
DEATHS 17 The death rate in adults with sickle cell disease who receive transfusions is 3 times higher than the general US population.
†Defined as serum ferritin levels >1500 ng/mL and transferrin saturation >50%. Without iron overload was defined as serum ferritin levels <100 and transferrin saturation <50%.2
A large study of >1,000 people with sickle cell anemia showed that kidney failure was responsible for 14% of deaths in people with sickle cell disease.
Ferriprox has not been proven to impact outcomes such as death, organ function, or symptoms such as pain episodes.
Iron chelation therapy is most beneficial when it is started early and taken as prescribed
The ASH guidelines recommend T2* MRI screening of the liver and heart be performed for sickle cell disease patients.
ASH guidelines recommend liver iron overload MRI T2* every 1 to 2 years
Liver iron overload should be checked by MRI every 1 to 2 years if you receive regular transfusions.
ASH guidelines recommend heart MRI T2* if you have:
- sickle cell disease with a high iron burden (liver iron content >15 mg/g [dry weight (dw)]) for 2 years or more
- evidence of end organ damage because of transfusional iron overload
- or evidence of cardiac dysfunction
Use the MRI T2* facility search to find the one nearest you >
Ferriprox is an oral medication that is taken either as a tablet or a liquid solution.
Your doctor may adjust your dose to minimize GI upset when first starting therapy and over time to help you receive the full benefit of Ferriprox treatment.1



Increasing the dose of Ferriprox from 75 mg/kg/day up to 99 mg/kg/day may improve efficacy in iron chelation.1,23
Yes. Ferriprox is suitable for patients with reduced kidney or liver† function. No change of the Ferriprox dose is required in patients with a mild to severe reduction in kidney function or a mild to moderate reduction in liver† function.
† Ferriprox was not studied in patients with severely reduced liver function.
Talk to your doctor about switching to the Ferriprox Twice-A-Day formulation >
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
During treatment, your doctor will also do monthly blood tests to check your liver function. They will also do blood tests to check your zinc levels before you start and during treatment with Ferriprox.
If you develop a fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking follow these 3 steps:
Stop the drug immediately
Seek medical attention immediately (i.e., go to the ER or your doctor)
Notify the ER provider or your doctor that you are taking a medication that can cause agranulocytosis
A wallet card with these three steps printed on it is included in every one of your Ferriprox medication shipments. To learn more or request a wallet card, call the Chiesi Total CareSM team at: 1-866-758-7071.
Ferriprox offers a choice of oral chelation formulations.†
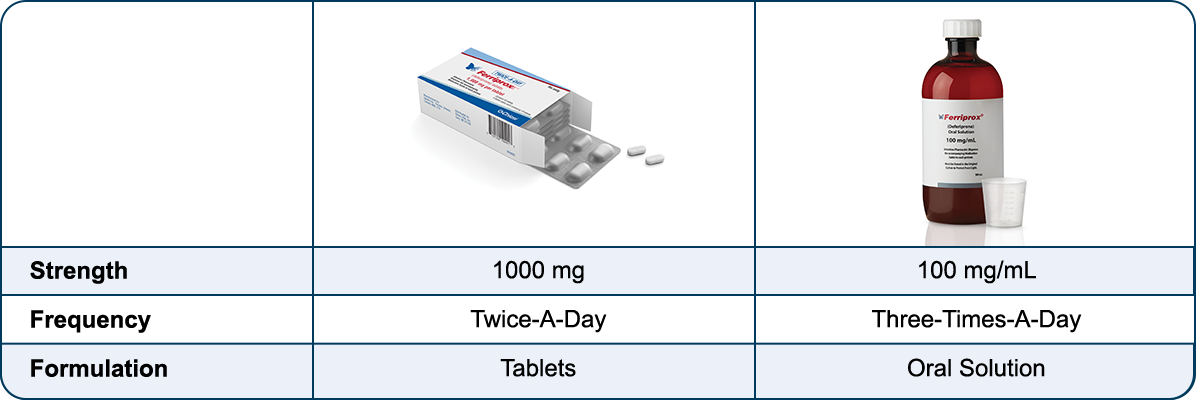


Talk to your healthcare provider about the formulation you prefer.
† 500 mg and 1000 mg Three-Times-A-Day tablets are still available. Talk to a Chiesi Total Care pharmacist for more information: 1-866-758-7071
Chiesi Total CareSM offers one-stop patient support. A single call to your dedicated Chiesi Total Care team is all it takes to guide you through the process of getting started on Ferriprox therapy. Visit chiesitotalcare.com or call 1-866-758-7071. We’re ready to help!
IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
