Sickle cell disease can lead to progressive organ damage1,2
Worsening of the condition is related to damage to multiple organs in the body starting in childhood1,3
Insert text here...
Complications of transfusional iron overload
Treatment with regular blood transfusions results in iron accumulation which can damage multiple organs such as the liver, heart, and endocrine system.4
Ferriprox has not been proven to impact outcomes such as death, organ functions, or symptoms such as pain episodes.
†Defined as serum ferritin levels >1500 ng/mL and transferrin saturation >50%. Without iron overload was defined as serum ferritin levels <100 and transferrin saturation <50%.5
Iron chelation is the primary treatment for transfusional iron overload7
The body normally excretes 1 to 2 mg of iron/day.7
Each blood transfusion adds 200 to 250 mg of iron to the body’s iron burden.4
One unit of blood can deliver >100X the normal daily iron absorbed8
It is important to keep track of all your blood transfusions, including the ones you might receive in the ER.
It is important to keep track of all your blood transfusions, including the ones you might receive in the ER.
In sickle cell disease, expert guidelines say you should start iron chelation when you:7
Insert text here...



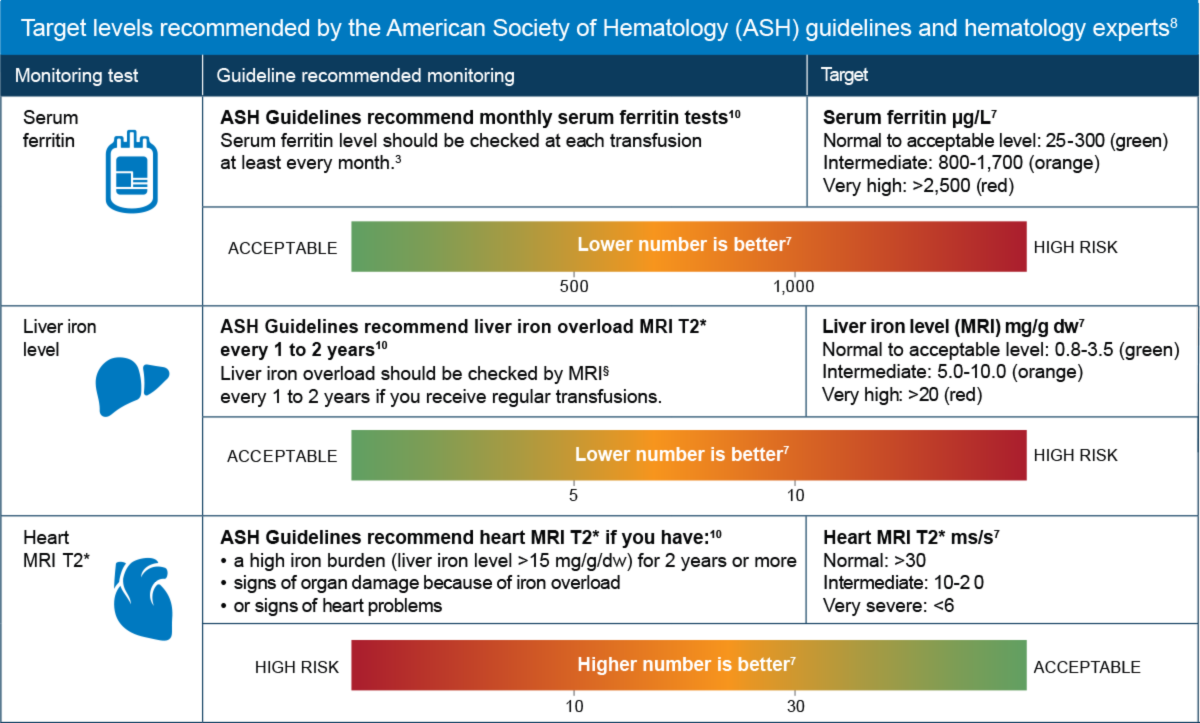
Iron monitoring in sickle cell disease
Serum ferritin level and heart and liver iron should all be checked regularly10
Insert text here...


IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
IMPORTANT SAFETY INFORMATION
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
What is the most important information I should know about Ferriprox?
Ferriprox can cause serious side effects, including a very low white blood cell count. One type of white blood cell that is important for fighting infections is called a neutrophil. If your neutrophil count is low (neutropenia), you may be at risk of developing a serious infection that can lead to death. Neutropenia is common with Ferriprox and can become severe in some people. Severe neutropenia is known as agranulocytosis. If you develop agranulocytosis, you will be at risk of developing serious infections that can lead to death.
Your healthcare provider will do a blood test before you start Ferriprox and regularly during treatment to check your neutrophil count. If you develop neutropenia, your healthcare provider should check your blood counts every day until your white blood cell count improves. Your healthcare provider may temporarily stop treatment with Ferriprox if you develop neutropenia or infection.
Stop taking Ferriprox and call your healthcare provider or get medical help right away if you develop any of these symptoms of infection: fever, sore throat or mouth sores, flu-like symptoms, or chills and severe shaking.
It is important for you to have your white blood cell count checked within 24 hours of developing symptoms of an infection to see if you have severe neutropenia (agranulocytosis). Do not delay getting medical care if you are unable to reach your healthcare provider.
What is Ferriprox® (deferiprone)?
Ferriprox® (deferiprone) is a prescription medicine used to treat iron overload from blood transfusions in people with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are for adults and children ≥8 years of age; Ferriprox Oral Solution is for patients ≥3 years of age.
It is not known if Ferriprox is safe and effective to treat iron overload due to blood transfusions:
- in people with myelodysplastic syndrome or Diamond Blackfan anemia
- in children less than 3 years of age
Do not take Ferriprox if you are allergic to deferiprone or any of the ingredients in Ferriprox.
Before you take Ferriprox, tell your healthcare provider about all of your medical conditions, including if you: have liver problems, are pregnant or plan to become pregnant. Ferriprox can harm your unborn baby. You should avoid becoming pregnant during treatment with Ferriprox. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Ferriprox. For females who are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Ferriprox. You should use effective birth control during treatment with Ferriprox and for at least 6 months after the last dose. For males with female partners who are able to become pregnant, you should use effective birth control during treatment with Ferriprox and for at least 3 months after the last dose. Talk to your doctor if you are breastfeeding or plan to breastfeed. It is not known if Ferriprox passes into your breast milk. Do not breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Avoid drinking alcohol during treatment with Ferriprox tablets (2 times a day). This may cause a faster release of the medicine.
What are other possible side effects of Ferriprox?
Ferriprox can cause serious side effects, including increased liver enzyme levels in your blood. Your healthcare provider should do blood tests to check your liver function before you start and then monthly during treatment with Ferriprox. Your healthcare provider may temporarily stop treatment with Ferriprox tablets if you develop increased liver enzyme levels and they continue to be increased.
Ferriprox can cause decreased levels of zinc in your blood. Your healthcare provider will do blood tests to check your zinc levels before you start and during treatment with Ferriprox and may prescribe a zinc supplement for you if your zinc levels are low.
The most common side effects of Ferriprox in people with thalassemia include nausea, vomiting, stomach-area (abdominal) pain, joint pain, abnormal liver function tests and low white blood cells.
The most common side effects of Ferriprox in people with sickle cell disease or other anemias include fever, stomach-area (abdominal) pain, bone pain, headache, vomiting, pain in arms or legs, sickle cell anemia with crisis, back pain, abnormal liver function tests, joint pain, mouth and throat pain, common cold, low white blood cells, cough and nausea.
Ferriprox may cause a change in urine color to reddish-brown. This is not harmful and is expected during treatment with Ferriprox.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
References: 1. Buchanan G, Vichinksy E, Krishnamurti L et al. Severe Sickle Cell Disease – Pathophysiology and Therapy. Biol Blood Marrow Transplant 2010;16(1):S64-7. 2. National Organization for Rare Disorders. Beta Thalassemia. Accessed online December 2021 at: https://rarediseases.org/rare-diseases/thalassemia-major/. 3. Evidence-based management of sickle cell disease: Expert panel report, 2014. National Heart Lung and Blood Institute. Accessed online January 13, 2021 at: https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease. 4. Hider RC, Hoffbrand AV. The role of deferiprone in iron chelation. N Engl J Med 2018;379:2140-50. 5. Ballas SK et al. The effect of iron chelation therapy on overall survival in sickle cell disease and ß-thalassemia: A systematic review. Am J Hematol 2018;93:943-52. 6. Cappellini MD, Cohen A, Porter J, et al., editors. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT)[Internet]. 3rd edition. Nicosia (CY): Thalassaemia International Federation; 2014. 7. Coates TD, Wood JC. How we manage iron overload in sickle cell patients. Br. J Haematol 2017;177(5):703-16. 8. Kessler CM et al. Iron chelation for iron overload secondary to transfusions of packed red blood cells. Advances in Hematology 2014; 12(3):184-5. 9. Standards of care guidelines for thalassemia. Children’s Hospital & Research Center Oakland. 2012. Available online at: https://thalassemia.com/documents/SOCGuidelines2012.pdf. 10. Chou ST et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood 2020;4(2):327-55.
