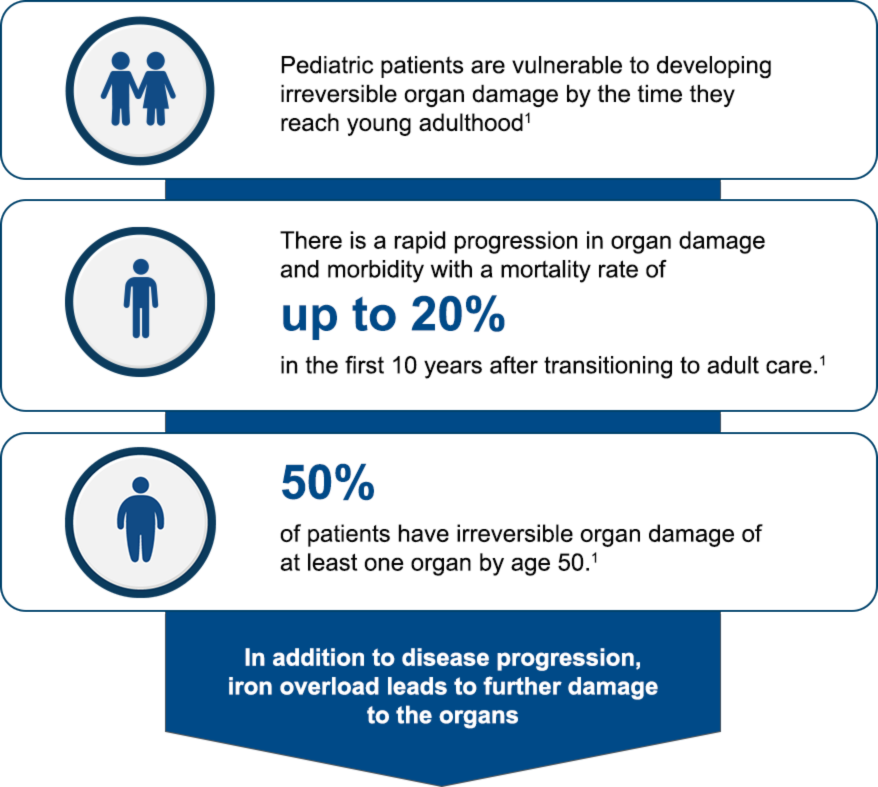
The body normally excretes 1 to 2 mg of iron/day.8
Each blood transfusion adds 200 to 250 mg of iron to the body’s iron burden.4
One transfusion can deliver >100X the normal daily iron absorbed9
This informational website is developed by Chiesi USA, and is intended only for HCPs that are residents of the United States


Insert text here...
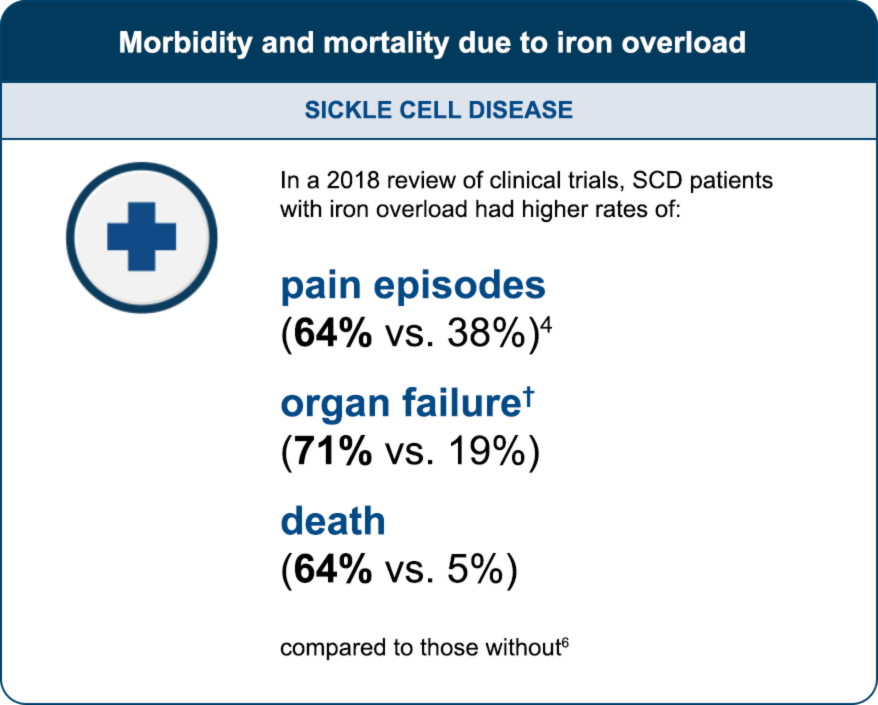


† Defined as serum ferritin levels >1500 ng/mL and transferrin saturation >50%. Without iron overload was defined as serum ferritin levels <100 and transferrin saturation <50%.6
Ferriprox has not been proven to impact outcomes such as death, organ function, or symptoms such as pain episodes.
One transfusion can deliver >100X the normal daily iron absorbed9
Insert text here...



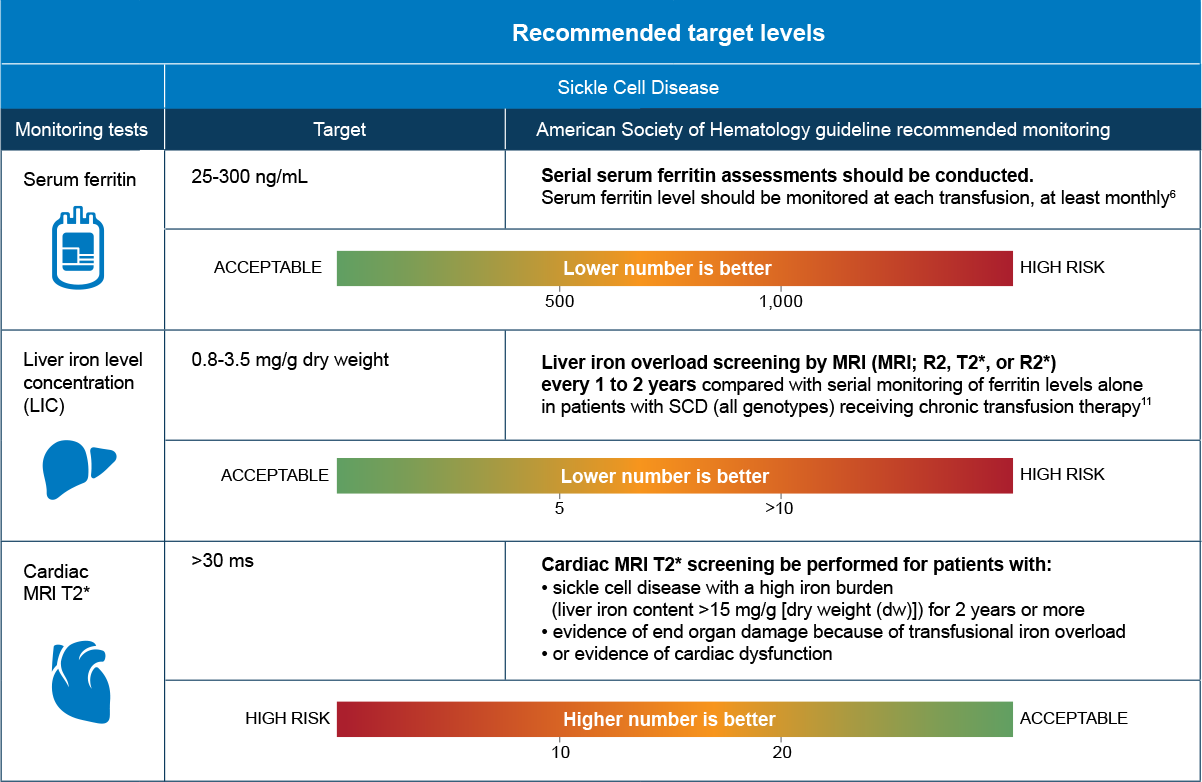
Treatment guidelines recommend preventing significant iron loading from the start.8,10
Insert text here...
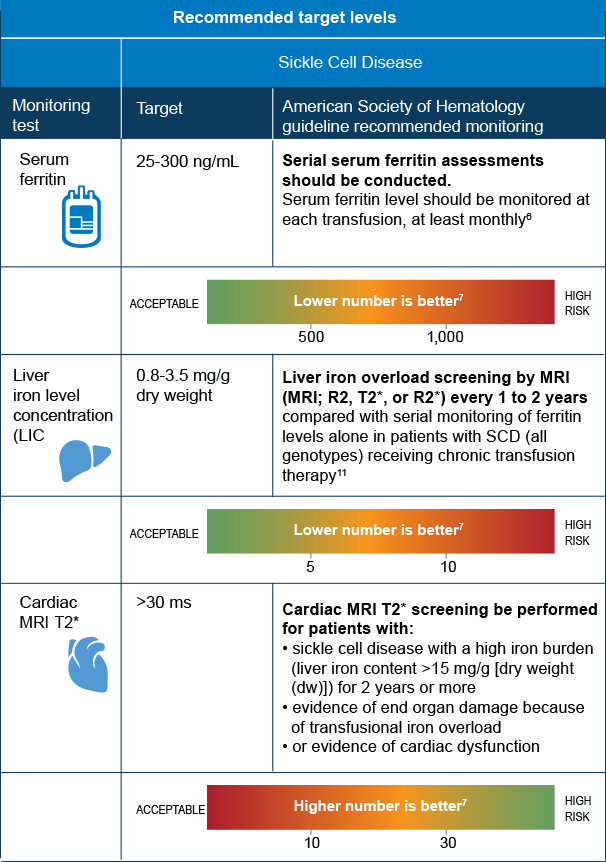

ms=milliseconds
MRI=magnetic resonance imaging
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:1
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Important Safety Information
Ferriprox is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulations.
In pooled clinical trials, 7.5% of 642 patients with thalassemia syndromes treated with Ferriprox developed increased ALT values. Four (0.62%) Ferriprox-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST. In pooled clinical trials, 7.7% of 196 patients with sickle cell disease or other anemias treated with Ferriprox developed increased ALT values. Monitor serum ALT values monthly during therapy with Ferriprox and consider interruption of therapy if there is a persistent increase in the serum transaminase levels. Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc annually, and supplement in the event of a deficiency.
Ferriprox can cause fetal harm. Advise females of reproductive potential to use an effective method of contraception during treatment with Ferriprox and for at least six months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Ferriprox and for at least three months after the last dose. Advise females not to breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Avoid co-administration of Ferriprox with other drugs known to be associated with neutropenia or agranulocytosis; however, if this is unavoidable, closely monitor the absolute neutrophil count. Avoid co-administration with UGT1A6 inhibitors. Allow at least a 4-hour interval between administration of Ferriprox and drugs or supplements containing polyvalent cations (e.g., iron, aluminum, or zinc).
The most common adverse reactions in patients with thalassemia (incidence ≥6%) are nausea, vomiting, abdominal pain, arthralgia, ALT increased and neutropenia. The most common adverse reactions in patients with sickle cell disease or other anemias (incidence ≥6%) are pyrexia, abdominal pain, bone pain, headache, vomiting, pain in extremity, sickle cell anemia with crisis, back pain, ALT increased, AST increased, arthralgia, oropharyngeal pain, nasopharyngitis, neutrophil count decreased, cough and nausea.
Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect, and it is not harmful.
Advise patients to avoid alcohol while taking Ferriprox tablets (twice-a-day). Consumption of alcohol while taking Ferriprox tablets (twice-a-day) may result in more rapid release of deferiprone.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:1
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Important Safety Information
Ferriprox is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulations.
In pooled clinical trials, 7.5% of 642 patients with thalassemia syndromes treated with Ferriprox developed increased ALT values. Four (0.62%) Ferriprox-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST. In pooled clinical trials, 7.7% of 196 patients with sickle cell disease or other anemias treated with Ferriprox developed increased ALT values. Monitor serum ALT values monthly during therapy with Ferriprox and consider interruption of therapy if there is a persistent increase in the serum transaminase levels. Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc annually, and supplement in the event of a deficiency.
Ferriprox can cause fetal harm. Advise females of reproductive potential to use an effective method of contraception during treatment with Ferriprox and for at least six months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Ferriprox and for at least three months after the last dose. Advise females not to breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Avoid co-administration of Ferriprox with other drugs known to be associated with neutropenia or agranulocytosis; however, if this is unavoidable, closely monitor the absolute neutrophil count. Avoid co-administration with UGT1A6 inhibitors. Allow at least a 4-hour interval between administration of Ferriprox and drugs or supplements containing polyvalent cations (e.g., iron, aluminum, or zinc).
The most common adverse reactions in patients with thalassemia (incidence ≥6%) are nausea, vomiting, abdominal pain, arthralgia, ALT increased and neutropenia. The most common adverse reactions in patients with sickle cell disease or other anemias (incidence ≥6%) are pyrexia, abdominal pain, bone pain, headache, vomiting, pain in extremity, sickle cell anemia with crisis, back pain, ALT increased, AST increased, arthralgia, oropharyngeal pain, nasopharyngitis, neutrophil count decreased, cough and nausea.
Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect, and it is not harmful.
Advise patients to avoid alcohol while taking Ferriprox tablets (twice-a-day). Consumption of alcohol while taking Ferriprox tablets (twice-a-day) may result in more rapid release of deferiprone.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
References: 1. Buchanan G, Vichinksy E, Krishnamurti L et al. Severe Sickle Cell Disease – Pathophysiology and Therapy. Biol Blood Marrow Transplant 2010;16(1):S64-7. 2. National Organization for Rare Disorders. Beta Thalassemia. Accessed online December 2021 at: https://rarediseases.org/rare-diseases/thalassemia-major/ 3. Evidence-based management of sickle cell disease: Expert panel report, 2014. National Heart Lung and Blood Institute. Accessed online January 13, 2021 at: https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease 4. Hider RC, Hoffbrand AV. The role of deferiprone in iron chelation. N Engl J Med 2018;379:2140-50. 5. Borgna-Pignatti C, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004;89:1187-1193. 6. Ballas SK et al. The effect of iron chelation therapy on overall survival in sickle cell disease and ß-thalassemia: A systematic review. Am J Hematol 2018;93:943-52. 7. Cappellini MD, Cohen A, Porter J, et al., editors. Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT)[Internet]. 3rd edition. Nicosia (CY): Thalassaemia International Federation; 2014. 8. Coates TD, Wood JC. How we manage iron overload in sickle cell patients. Br. J Haematol 2017;177(5):703-16. 9. Kessler CM et al. Iron chelation for iron overload secondary to transfusions of packed red blood cells. Advances in Hematology 2014; 12(3):184-5. 10. Standards of care guidelines for thalassemia. Children’s Hospital & Research Center Oakland. 2012. Available online at: https://thalassemia.com/documents/SOCGuidelines2012.pdf. 11. Chou ST et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood 2020;4(2):327-55.