The Ferriprox safety profile has been established in 70+ thalassemia studies worldwide1
Ferriprox has 20 years of worldwide post-marketing experience in thalassemia; (10 in the US) and over 113,000+ patient-years of use.1
Agranulocytosis and Neutropenia
In clinical trials of 196 Ferriprox-treated patients with sickle cell disease or other anemias:1
- agranulocytosis occurred in 1.5% of patients
Fatal agranulocytosis can occur with Ferriprox use. Ferriprox can also cause neutropenia, which may foreshadow agranulocytosis.
Most common adverse reactions occurring in ≥6% of patients with sickle cell disease and other anemias treated with Ferriprox in clinical trials2† | |
|---|---|
| Adverse reaction | % Patients (n=152) |
| Pyrexia | 28 |
| Abdominal pain | 26 |
| Bone pain | 25 |
| Headache | 20 |
| Vomiting | 19 |
| Pain in extremity | 18 |
| Sickle cell anemia with crisis | 17 |
| Back pain | 13 |
| Alanine aminotransferase increased | 12 |
| Aspartate aminotransferase increased | 11 |
| Arthralgia | 10 |
| Oropharyngeal pain | 10 |
| Nasopharyngitis | 9 |
| Neutrophil count decreased | 8 |
| Cough | 8 |
| Nausea | 7 |
† Adverse reaction frequencies are based on adverse events reported regardless of relationship to study drug.
‡ Grouped term
To minimize gastrointestinal upset when first starting therapy, dosing can start at 45 mg/kg/day and increase weekly by 15 mg/kg/day until the full prescribed dose is achieved.2
Monitoring your patients on Ferriprox is straightforward:2
Absolute neutrophil count (ANC)
Insert text here...
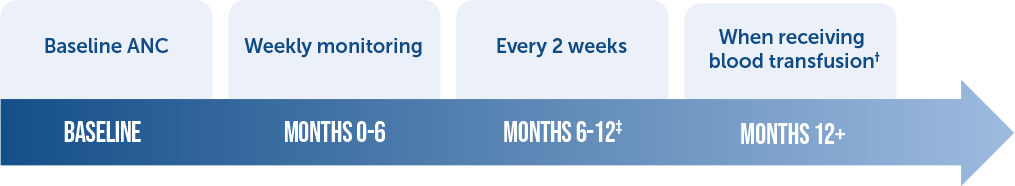


§ For patients whose Ferriprox has not been interrupted due to any decrease in the neutrophil count the frequency of ANC monitoring may be extended.
¶ After one year of therapy: Monitor ANC every two to four weeks (or at the patient’s blood transfusion interval) in patients that have not experienced an interruption due to any decrease in ANC.
Reduction in the frequency of ANC monitoring should be considered on an individual patient basis, according to the health care provider’s assessment of the patient’s understanding of the risk minimization measures required during therapy.
Serum liver enzymes
Monthly, on therapy
Increased ALT levels were observed in clinical trials. Consider interruption of therapy if there is a persistent increase in serum transaminase levels.
Plasma zinc concentration
Annually, on therapy
Decreased plasma zinc concentrations have been observed in patients on Ferriprox
IMPORTANT SAFETY INFORMATION
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
- Ferriprox can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis.
- Measure the absolute neutrophil count (ANC) before starting Ferriprox and monitor regularly while on therapy.
- Interrupt Ferriprox therapy if neutropenia develops.
- Interrupt Ferriprox if infection develops, and monitor the ANC more frequently.
- Advise patients taking Ferriprox to report immediately any symptoms indicative of infection.
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Important Safety Information
Ferriprox is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulations.
In pooled clinical trials, 7.5% of 642 patients with thalassemia syndromes treated with Ferriprox developed increased ALT values. Four (0.62%) Ferriprox-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST. In pooled clinical trials, 7.7% of 196 patients with sickle cell disease or other anemias treated with Ferriprox developed increased ALT values. Monitor serum ALT values monthly during therapy with Ferriprox and consider interruption of therapy if there is a persistent increase in the serum transaminase levels. Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc annually, and supplement in the event of a deficiency.
Ferriprox can cause fetal harm. Advise females of reproductive potential to use an effective method of contraception during treatment with Ferriprox and for at least six months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Ferriprox and for at least three months after the last dose. Advise females not to breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Avoid co-administration of Ferriprox with other drugs known to be associated with neutropenia or agranulocytosis; however, if this is unavoidable, closely monitor the absolute neutrophil count. Avoid co-administration with UGT1A6 inhibitors. Allow at least a 4-hour interval between administration of Ferriprox and drugs or supplements containing polyvalent cations (e.g., iron, aluminum, or zinc).
The most common adverse reactions in patients with thalassemia (incidence ≥6%) are nausea, vomiting, abdominal pain, arthralgia, ALT increased and neutropenia. The most common adverse reactions in patients with sickle cell disease or other anemias (incidence ≥6%) are pyrexia, abdominal pain, bone pain, headache, vomiting, pain in extremity, sickle cell anemia with crisis, back pain, ALT increased, AST increased, arthralgia, oropharyngeal pain, nasopharyngitis, neutrophil count decreased, cough and nausea.
Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect, and it is not harmful.
Advise patients to avoid alcohol while taking Ferriprox tablets (twice-a-day). Consumption of alcohol while taking Ferriprox tablets (twice-a-day) may result in more rapid release of deferiprone.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
IMPORTANT SAFETY INFORMATION
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
WARNING: AGRANULOCYTOSIS AND NEUTROPENIA
- Ferriprox can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis.
- Measure the absolute neutrophil count (ANC) before starting Ferriprox and monitor regularly while on therapy.
- Interrupt Ferriprox therapy if neutropenia develops.
- Interrupt Ferriprox if infection develops, and monitor the ANC more frequently.
- Advise patients taking Ferriprox to report immediately any symptoms indicative of infection.
Indication
Ferriprox® (deferiprone) is an iron chelator indicated for the treatment of transfusional iron overload in patients with:1
- thalassemia syndromes
- sickle cell disease or other anemias
Ferriprox Tablets are indicated in adult and pediatric patients ≥8 years of age; Ferriprox Oral Solution is indicated in patients ≥3 years of age.
Limitations of Use:
Safety and effectiveness have not been established for the treatment of transfusional iron overload in patients with myelodysplastic syndrome or in patients with Diamond Blackfan anemia.
Important Safety Information
Ferriprox is contraindicated in patients with known hypersensitivity to deferiprone or to any of the excipients in the formulations.
In pooled clinical trials, 7.5% of 642 patients with thalassemia syndromes treated with Ferriprox developed increased ALT values. Four (0.62%) Ferriprox-treated subjects discontinued the drug due to increased serum ALT levels and 1 (0.16%) due to an increase in both ALT and AST. In pooled clinical trials, 7.7% of 196 patients with sickle cell disease or other anemias treated with Ferriprox developed increased ALT values. Monitor serum ALT values monthly during therapy with Ferriprox and consider interruption of therapy if there is a persistent increase in the serum transaminase levels. Decreased plasma zinc concentrations have been observed on deferiprone therapy. Monitor plasma zinc annually, and supplement in the event of a deficiency.
Ferriprox can cause fetal harm. Advise females of reproductive potential to use an effective method of contraception during treatment with Ferriprox and for at least six months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with Ferriprox and for at least three months after the last dose. Advise females not to breastfeed during treatment with Ferriprox and for at least 2 weeks after the last dose.
Avoid co-administration of Ferriprox with other drugs known to be associated with neutropenia or agranulocytosis; however, if this is unavoidable, closely monitor the absolute neutrophil count. Avoid co-administration with UGT1A6 inhibitors. Allow at least a 4-hour interval between administration of Ferriprox and drugs or supplements containing polyvalent cations (e.g., iron, aluminum, or zinc).
The most common adverse reactions in patients with thalassemia (incidence ≥6%) are nausea, vomiting, abdominal pain, arthralgia, ALT increased and neutropenia. The most common adverse reactions in patients with sickle cell disease or other anemias (incidence ≥6%) are pyrexia, abdominal pain, bone pain, headache, vomiting, pain in extremity, sickle cell anemia with crisis, back pain, ALT increased, AST increased, arthralgia, oropharyngeal pain, nasopharyngitis, neutrophil count decreased, cough and nausea.
Inform patients that their urine might show a reddish/brown discoloration due to the excretion of the iron-deferiprone complex. This is a very common sign of the desired effect, and it is not harmful.
Advise patients to avoid alcohol while taking Ferriprox tablets (twice-a-day). Consumption of alcohol while taking Ferriprox tablets (twice-a-day) may result in more rapid release of deferiprone.
Please see Full Prescribing Information, including boxed WARNING, and Medication Guide.
References: 1. Data on file. 2. Ferriprox® (deferiprone) Prescribing Information. Chiesi, November 2021.
